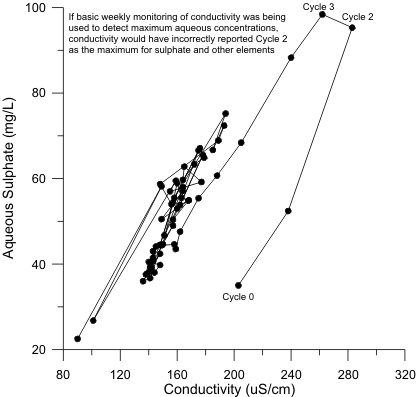
Figure 1. Example Correlation of Sulphate and Conductivity, Showing Maximum Conductivity is Not Necessarily Maximum Sulphate.
MDAG.com Internet Case Study 24
Errors from Sampling Humidity Cells Every Second Cycle
by K.A. Morin and N.M. Hutt
© 2007 Kevin A. Morin and Nora M. Hutt
Click here for a PDF version of this MDAG.com Internet Case Study 24
Abstract
An increasingly common trend in humidity-cell testing is not to analyze every weekly cycle before geochemical stability (often around 30-40 weeks into testing). Based on sixteen randomly chosen humidity cells from three sites, sampling and analyzing every second-week (biweekly) cell effluent can lead to significant errors in statistical calculations of minimums, averages, and maximum values. As a general indication of potential errors of biweekly analysis, we compared statistics from even-numbered cycles to those from odd-numbered cycles.
If maximum concentrations are used for “reasonable worst case” predictions, then biweekly sampling of cells could underestimate the real maximum concentration by one order of magnitude or more. However, for a particular cell, there is no way to determine in advance if the maximum was captured by biweekly sampling and, if not, how much higher the real maximum might be. Not even basic weekly monitoring of pH and conductivity would necessarily help, particularly for minor elements.
Average concentrations based on even-cycled and odd-cycled biweekly analyses of sixteen cells differed by more than a factor of two. A larger set of cells would probably show that average concentrations based on biweekly analyses could vary by larger factors.
Therefore, humidity cells should be sampled and analyzed fully every cycle until they are relatively stable geochemically, if good accuracy and predictive capability are needed. This typically requires 30-40 weeks of analyses, but shorter and longer periods to stability have been encountered.
Introduction
We have noticed an increasingly common trend not to analyze humidity cells every cycle, normally every week. We believe every cycle should be analyzed until a cell is generally stable geochemically, often around 30-40 weeks into testing (Morin and Hutt, 1997, 1999, and 2001).
The desire to reduce sampling and analysis is understandable, because money can be saved over the many months or years of testing. However, substantial errors in statistical values, like averages, minimums, and maximums, due to less frequent analysis would make the cell results unreliable and thus offset the economic benefit.
Therefore, in this case study, we:
1) chose 16 humidity cells from three sites at random to represent a range of acidic and near-neutral conditions,
2) determined minimum concentrations, average concentrations, and maximum concentrations using (a) only even numbered weekly cycles and (b) only odd numbered weekly cycles, to simulate biweekly analyses, and
3) calculated the ratios of the even-cycle statistics to the odd-cycle statistics and vice versa.
For example, a ratio of 1.5 for maximum aqueous sulphate concentrations means that the even-cycle maximum concentration was 1.5 times higher than the odd-cycle concentration, or vice versa. All ratios were adjusted so that they were always ≥ 1.0, because it was irrelevant whether the odd-cycle value was greater or less than the even-cycle value in a particular cell.
There is sometimes a belief that minimal analysis, like weekly conductivity and pH, would be sufficient to detect anomalous values and thus trigger additional analyses. This assumption is also examined in this case study.
Database
We arbitrarily selected three sites with a range of pH and acidity, from strongly acidic to near neutral. This provided sixteen humidity cells (Table 1), from which we calculated even-cycle and odd-cycle statistics for minimum, average, and maximum concentrations. This included dozens of elements. We were particularly interested in the average values, because they have been used for “average” predictions of future minesite-drainage chemistry. We were also interested in maximum values, because they have been used for “reasonable worst case” predictions.
Elements with weekly concentrations frequently below detection (more than half the weekly cycles for a cell) were ignored. This was because their minimum, average, and maximum values would be similar or identical, simply due to the artifact of the detection limit.
These sixteen cells should not be taken as a statistically reliable indication of the biweekly sampling errors. They are used, however, to highlight the potential severity of the errors in such sampling.
In other words, this paper does not provide a statistical “correction factor” to adjust errors from the biweekly sampling. In fact, such a factor does not exist. This paper shows that a correction factor would depend on whether even-cycle or odd-cycle analysis is executed, without any ability to know in advance which sampling would be more accurate. This adds to our concern about reduced sampling of humidity cells until they generally stabilize.
Table 1. Summary of the Sixteen Humidity Cells Used in This MDAG Case Study |
|||||
Site |
Cell |
Number of Weekly Cycles |
Average pH |
Average Sulphate (mg/L) |
Average Acidity (mg/L) |
1 |
1 |
52 |
3.7 |
211 |
169 |
2 |
52 |
4.6 |
68 |
27 |
|
3 |
52 |
7.0 |
17 |
3 |
|
4 |
52 |
7.6 |
54 |
3 |
|
5 |
52 |
7.7 |
25 |
2 |
|
2 |
1 |
52 |
3.5 |
315 |
305 |
2 |
43 |
8.0 |
58 |
4 |
|
3 |
43 |
7.9 |
49 |
3 |
|
4 |
43 |
8.4 |
19 |
2 |
|
5 |
43 |
8.0 |
11 |
2 |
|
3 |
1 |
41 |
2.5 |
585 |
611 |
2 |
41 |
2.1 |
1466 |
1594 |
|
3 |
41 |
2.2 |
1364 |
1298 |
|
4 |
41 |
2.2 |
1039 |
1022 |
|
5 |
41 |
2.1 |
1865 |
1939 |
|
6 |
41 |
2.1 |
1446 |
1467 |
|
Error in Maximum Concentrations with Biweekly Analysis
If weekly effluent from a humidity cell is analyzed every second week, there is a 50% probability that the real weekly maximum concentration will be measured. There is a 50% probability the real maximum will be higher than the biweekly maximum value by an unknown amount. Simplistically, “either you caught the maximum, or it is higher than what you think”. This is enough of a concern to justify analysis of every cycle.
An additional issue is: how much higher could the maximum concentration be if it were not caught in the biweekly analyses? The sixteen humidity cells give some idea.
In the worst case, one near-neutral cell showed that the maximum concentration of sulphate, if missed by the biweekly sampling, was a factor of 2.4 greater than the measured value. That is, the ratio of maximum concentrations was 2.4 from the even-cycle and odd-cycle sets. A few other cells produced ratios close to 2.0, and the smallest ratio was a negligible 1.03. There is no reason to expect these sixteen cells produced the largest possible discrepancy of a factor of 2.4, so larger errors can be expected but cannot be predicted in advance. Therefore, there would be no way of knowing in advance whether biweekly sampling had captured the maximum sulphate concentration or had underestimated it by a small amount, by 2.4 times, or even more.
Similarly, there was a 50% probability that the maximum concentrations of acidity and alkalinity could be under-reported by factors of 2.7 and 2.4 based only on the sixteen cells of Table 1. In contrast, dissolved metals and other elements were generally worse, with 50% probabilities that biweekly sampling would underestimate their maximum levels typically by more than a factor of two and up to a factor of 19.
Therefore, if maximum concentrations are used for “reasonable worst case” predictions, then biweekly sampling of cells could underestimate the real maximum concentration by an order of magnitude or more. However, for a particular cell, there is no way to determine if the maximum was captured by biweekly sampling and, if not, how much higher the real maximum might be. Not even basic weekly monitoring of pH and conductivity would necessarily help, as explained below.
Error in Average Concentrations with Biweekly Analysis
As explained above, there was a 50% probability of measuring the actual maximum concentration for a particular element using biweekly cell analyses, and a 50% probability the real maximum could be more than one order of magnitude higher. Average concentrations do not follow this rule. There is a small probability, from coincidence, that the even-cycle average would equal the odd-cycle average. However, unlike maximum values, average values are “smoothed” out over months of cell testing so that missing weeks and missing maximums do not always greatly affect the long-term average. Thus, there is little probability that average values of even-cycle and odd-cycle analyses would be equal, but a high expectation of not differing by much. The sixteen cells give some idea of what “much” might be.
For average sulphate concentrations, the largest ratio was 1.3, meaning the even-cycle and odd-cycle averages from the sixteen cells differed by factors of less than 1.4. The largest ratios for acidity and alkalinity were 1.3 (like sulphate) and 1.8. For dissolved elements, roughly one-half had ratios close to and above 2.0, to a maximum of 3.5.
Therefore, average concentrations based on even-cycled and odd-cycled biweekly analyses of sixteen cells can differ by more than a factor of two. A larger set of cells would probably show that average concentrations based on biweekly analyses could vary by larger factors.
Detection of Maximum Values by Basic Weekly Monitoring of pH and Conductivity
Basic weekly sampling for easily (cheaply) measured parameters like pH and conductivity is sometimes assumed to show when a peak concentration occurred. This is based on the assumption that pH and conductivity have perfect 1:1 correlation for all major and minor elements. There is no reason to expect such a correlation for minor elements, unless there are major geochemical changes, so basic monitoring would not necessarily reveal peaks in trace metals.
Conductivity can reveal major changes in the dominant cations and anions. For example, sulphate can correlate with conductivity, although the highest conductivity may not be the highest sulphate (Figure 1).
Therefore, basic weekly monitoring of pH and conductivity may generally correlate with major cations and anions, but the correlation may not be sufficient to identify the maximum concentration. Furthermore, minor elements do not necessarily correlate strongly with pH and conductivity, especially over relatively minor changes, so peak values in minor elements also cannot be reliably detected by basic weekly monitoring.
Conclusion
Humidity cells should be sampled and analyzed fully every cycle until they are relatively stable geochemically, if good accuracy and predictive capability are needed. This typically requires 30-40 weeks of analyses, but shorter and longer periods to stability have been encountered.
References
Morin, K.A., and N.M. Hutt. 2001. Environmental Geochemistry of Minesite Drainage: Practical Theory and Case Studies - Digital Edition. MDAG Publishing (www.mdag.com), Surrey, Canada. ISBN 0-9682039-1-4.
Morin, K.A., and N.M. Hutt. 1999. Humidity Cells: How Long? How Many? Proceedings of Sudbury ‘99, Mining and the Environment II, Volume 1, p.109-117, Sudbury, Canada, September 13-15.
Morin, K.A., and N.M. Hutt. 1997. Environmental Geochemistry of Minesite Drainage: Practical Theory and Case Studies. MDAG Publishing (www.mdag.com), Surrey, Canada. ISBN 0-9682039-0-6.

© 2007 Kevin A. Morin and Nora M. Hutt
For more case studies, see Environmental Geochemistry of Minesite
Drainage: Practical Theory and Case Studies.
Created by K.Morin